 Uzma Hasan, MD
Uzma Hasan, MD
Clinical Associate Professor, Rutgers NJ Medical School
Division Director, Pediatric Infectious Diseases,
Cooperman Barnabas Medical Center
 Rachel Meyers, PharmD
Rachel Meyers, PharmD
Pediatric Clinical Pharmacist,
Cooperman Barnabas Medical Center
 Jessica Lise, PharmD, BCPPS
Jessica Lise, PharmD, BCPPS
Pediatric Clinical Specialist, Department of Pharmacy,
Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson University Hospital
- RSV is a respiratory virus that causes acute respiratory infection in all ages.
- Most infants will experience upper respiratory infection, 20-30% develop bronchiolitis
- 1-3% of children will require hospitalization for RSV in the first 12 months of life, highest hospitalization rates between 30-60 days of age.
- Increased risk of severe illness in infants born before 29 weeks of gestation, CLD (chronic lung disease of prematurity, certain types of congenital heart disease, immunodeficiency.
- Spread: direct/close contact with contaminated secretions, fomites.
RSV Vaccine for Pregnant Women
- ACIP meeting 9/22/23 met to review Abrysvo, Pfizer’s respiratory syncytial virus (RSV) vaccine (“RSVpreF”).
- 0.5ml IM x1 dose (120ug)
- Given atleast 2 weeks prior to delivery reduces significant LRTI in infant by 70%
- Seasonal administration: September through January
- Vaccine is given between 32-36 weeks GA
- If the mother was vaccinated during a previous pregnancy, CDC does not recommend another dose for subsequent pregnancies.
- In the phase 3 clinical trial:
- maternal RSV vaccine reduced the risk of the baby being hospitalized for RSV by 68% and reduced risk of having a healthcare visit for RSV by 57% within 3 months after birth.
- RSV vaccine reduced the risk of the baby being hospitalized for RSV by 57% 6 months after birth and reduced risk of having a healthcare visit for RSV by 51% within 6 months after birth.
- The maternal RSV vaccine reduced the risk of severe infant outcomes caused by RSV, including development of hypoxia, need for mechanical ventilation or admission to an intensive care unit (ICU), by 82% within 3 months and by 69% within 6 months after birth.
Monoclonal Antibody Against RSV: Nirsevimab (Beyfortus™)
- Approved by FDA on July 17, 2023
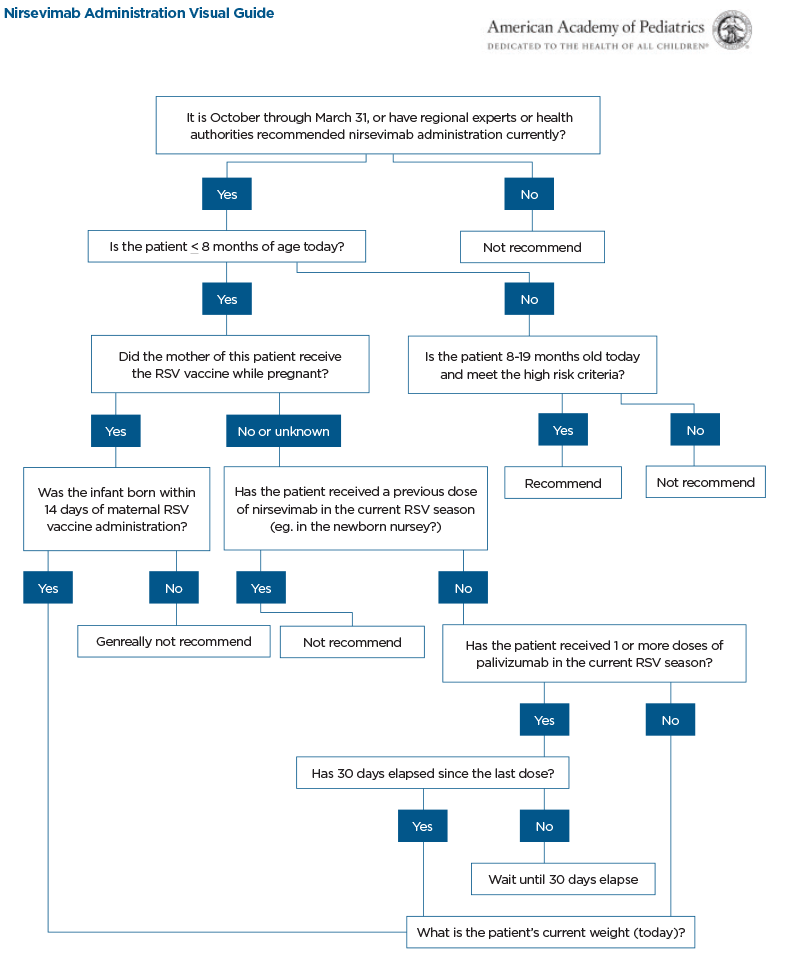
- Nirsevimab is a long-acting monoclonal antibody product intended for use in newborns and infants to protect against (medically attended) RSV. Nirsevimab is recommended for:
- All infants younger than 8 months born during or entering their first RSV season
- Infants and children aged 8 through 19 months who are at increased risk of severe RSV disease and entering their second RSV season
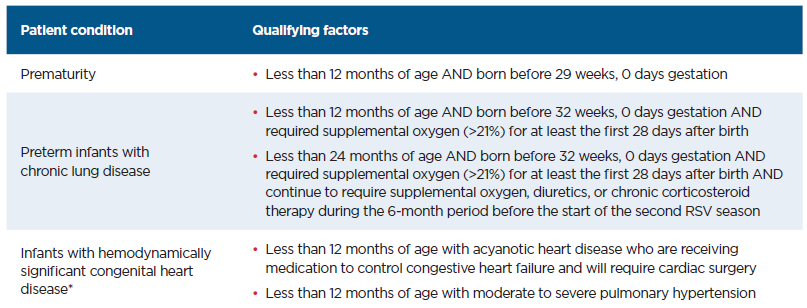
- High risk infants are those with: Chronic lung disease of prematurity requiring medical support in 6 months prior to RSV season, severe immunocompromised, Cystic fibrosis, American Indian/Alaskan Native children
- If mother received RSV vaccine >2 weeks prior to delivery, Nirsevimab is not indicated; however it may be considered for infants born to a vaccinated mother in rare circumstances:
- Inadequate maternal response to vaccination suspected.
- Infants who experience loss of maternal antibodies (e.g. those who have undergone ECMO, bypass)
- Infant with substantially increased risk for severe disease
- Optimal Timing of Nirsevimab: within 1 week of delivery
- Infants weighing <5 kg: 50 mg dose (purple plunger rod)
- Infants weighing ≥5 kg: 100 mg dose (light blue plunger rod)
- High risk are infants entering second RSV season should get a second dose of Nirsevimab between ages 8-19 months, single dose 200mg ( 2 shots I00mg IM)
- In phase 3 clinical trials, Nirsevimab efficacy against RSV-associated lower respiratory tract infection with hospitalization was 81% (95% CI = 62%–90%) through 150 days after receipt
- Nirsevimab effectiveness was 90% against RSV-associated hospitalization in infants in their first RSV season. Median time from receipt of nirsevimab to symptom onset was 45 days (IQR = 19–76).
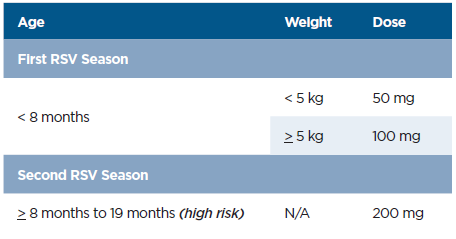
*Patients who undergo cardiopulmonary bypass after their dose will need an extra dose, per guidelines
Nirsevimab Administration Visual Guide
Synagis (Palivizumab)
- Palivizumab is a monoclonal immunoglobulin (Ig) G1K antibody produced by recombinant DNA technology.
- The antibody binds to RSV F protein’s antigenic epitope region, prevents viral entry into cells, prevents RSV infected cells from fusing with one another; hence Palivizumab reduces viral activity and prevents virus from invading host cells in airway.
- Given intramuscularly at a dose of 15 mg/kg, once every 30 days x 5 doses during RSV season to those children who are eligible to receive.
- If any infant receiving monthly prophylaxis experiences a breakthrough RSV infection, monthly prophylaxis should be discontinued because of the low likelihood of an RSV hospitalization following a second infection in the same season (<0.5%).
- Synagis reduced RSV hospitalizations in preterm infants with and without BPD by 55%.
Synagis (Palivizumab) Eligibility Criteria
Nirsevimab vs Palivizumab Guidance
- If no Nirsevimab available: high risk infants should receive Palivizumab in first and second year of life as recommended until Nirsevimab available.
- If Nirsevimab is administered, palivizumab should not be administered later that season.
- If palivizumab was administered initially for the season and <5 doses were administered, the infant should receive 1 dose of nirsevimab. No further palivizumab should be administered.
- No minimum interval between last dose of palivizumab and the dose of Nirsevimab.
- Protection from palivizumab wanes after 30 days, nirsevimab should be administered no later than 30 days after the last palivizumab dose, when possible.
- Palvizumab given season 1, and Nirsevimab available for season 2, use Nirsevimab.
The ASP, representing pediatric providers, including pediatric infectious disease specialists and pharmacists, exchange ideas, discuss case management strategies and develop and implement guidelines to be shared system wide, as well as serve as a resource for community physicians.
For more educational information, research and best practices from the Children’s Health Network at RWJBarnabas Health, visit rwjbh.org/childrenshealthresearch.
Resources
Nirsevimab Frequently Asked Questions (aap.org)
Nirsevimab Administration (aap.org)
Nirsevemab-Visual-Guide_Sept2023.pdf (aap.org)
https://www.cdc.gov/rsv/research/rsv-net/dashboard.html
Red book https://publications.aap.org/redbook/book/347/chapter/5755493/Respiratory-Syncytial-Virus?autologincheck=redirected
https://publications.aap.org/pediatrics/article/134/2/415/33013/Updated-Guidance-for-Palivizumab-Prophylaxis-Among
https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/pregnant-people.html#:~:text=Overview,is%20best%20for%20their%20family.
https://www.nejm.org/doi/full/10.1056/NEJMoa2216480 https://www.nejm.org/doi/full/10.1056/NEJMoa2110275